
Last September, science blogs breathlessly reported (if a blog can be said to be breathless) the “discovery of life on Venus”. Here was the lede from the European Association of Geochemistry:
“Scientists find gas linked to life in atmosphere of Venus”
As always, we parse the lede. The “gas” is phosphine (PH3), that is, three hydrogen atoms bonded to one phosphorus atom. The “find” was based on data collected from radio telescopes, manipulated to remove noise, and giving a signal proposed to arise from PH3. The “links to life” referred to some Earth organisms that make PH3, specifically, organisms that live in oxygen-scarce environments, which are overall “reducing”, as we chemists say. “Reducing” does not mean that they are losing weight; rather that it means that the ratio of hydrogen atoms to oxygen atoms is larger than it is in water.
The original paper was published in Nature Astronomy, one of the “Baby Natures” that Elsevier has spun off to monetize the “Nature” brand in scientific publishing (other examples are Nature Chemistry, Nature Biotechnology, and Nature Goat-herding).
Now, one should never spend much time throwing good thought at bad data. In a back-and-forth, some now suggest that the data manipulation process created a signal for PH3 that is not there; the data manipulators have responded by insisting the opposite.
However, since we are eager to increase the use of Aristotelian logic in astrobiology, and this provides a useful example towards that goal, let us assume that phosphine is there. The “syllogism” that one might extract from the Guardian reads:
- Some life on Earth generates phosphine
- Phosphine is found in the Venus atmosphere
- Therefore, Venus holds phosphine-generating life
Well, of course, that is no syllogism that Aristotle would recognize. But we might adjust it a bit:
- Phosphine can be found in the Venusian atmosphere if and only if Venus holds phosphine-generating life
- Phosphine is found in the Venus atmosphere
- Therefore, Venus holds phosphine-generating life
Better. The Guardian report quotes Jane Greaves, an astronomy professor at Cardiff University, who led the team making the report. She offered some support for the major premise. “[All] the geological and photochemical routes we can think of are far too underproductive to make the phosphine we see.”
This is called “biology in the gaps” thinking. It is analogous to the “God in the gaps” view of some creationists. If you cannot explain something that you see in the natural world, then God did it. Here, if you cannot explain something that you see on an alien world, then alien life did it.
William Bains, an outstanding chemist with whom we have worked previously on exotic biochemistries, elaborated chemically. William did not explain why Venusian PH3 must have come from life, but he did write out this helpful diagram that suggest how PH3 might come from life.
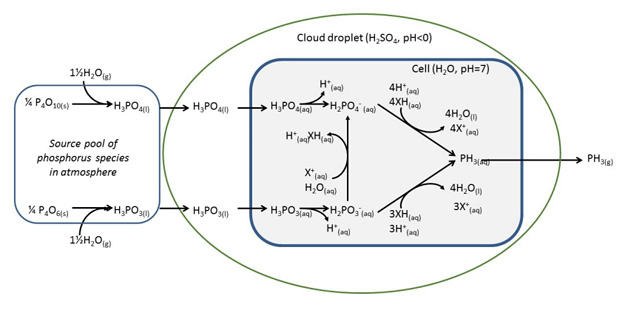
College level biochemistry textbooks hold this kind of diagram, so it seems impressive enough. However, it has a few omissions.
First, the clouds above Venus do indeed contain droplets of 90% sulfuric acid (H2SO4), and the pH of those droplets is indeed less than zero. However, physical organic chemistry offers something called the “Hammett Acidity Function“. Summarized, as the amount of sulfuric acid increases towards 100%, the effective pH of the medium drops very fast. Thus, a 10% solution of sulfuric acid is much more acidic than a 1% solution. And a 90% solution of sulfuric acid is, well, very very acidic.
Sulfuric acid at this concentration also seeks water very aggressively. Indeed, sulfuric acid extracts water from carbohydrates. Youtube has some nice videos. So the barrier that separates cell water at pH 7 from 90% H2SO4, the rounded blue rectangle in the diagram, if it exists, would be an exceptional material.
Further, the diagram focuses on the origin of “phosphorus species in [the] atmosphere” (P). The atmosphere where the phosphorus species noted are associated with 90% sulfuric acid (H2SO4). But phosphorus is hardly the atom that should worry us. The real question when in making PH3, in this atmosphere asks: Where do the H atoms, the hydrogen atoms, come from?
The diagram has them coming from water. But there is no water in any terran sense of the term, not in 90% H2SO4. You must get three the hydrogens from H2SO4. With six electrons to go with them.
Now, we have a problem saying that one cannot think of a “chemical” way to get phosphine (photochemical or geochemical) , so it must be made “by biology”. Biology is also just chemistry, different only because biological chemistry has access to Darwinian evolution.
For example, on the surface of Earth, hydrogen is also scarce. Its best source here actually is water, since we are not immersed in 90% H2SO4. Water is where plants get hydrogen to put into carbohydrates (which we animals eat). But plants get hydrogen from water using photochemistry. And a few minerals.
What makes biology special is that it can access Darwinian evolution. This means that if we want to evaluate the proposition in the Aristotelian syllogism that Venusian life must generate the PH3 that is observed (or not), we must ask how PH3 generation creates fitness. How does it help a Venusian survive, get married (or, at least, select a mate), and have children?
Now, the terran organisms that make PH3 do so in environments that are rich in hydrogen, and scarce in oxygen. They are reducing. H >>> O. Therefore, they take water (two hydrogens and one oxygen), grab the scarce oxygen for their personal use, combine the hydrogen with phosphorus, and throw the PH3 away. That is how the generation of PH3 makes them fit: In an environment where oxygen is scarce, mates love a partner who is rich in oxygen. There are human analogies.
Now, the Venusian atmosphere is no more “oxygen starved” than the atmosphere over the Amazon rainforest. On Venus, the oxygen is mostly in carbon dioxide (which has no useful hydrogen to offer). Yes, it might be able to extract hydrogen from sulfuric acid, H2SO4. But a bachelor Venusian who then throws away the hydrogen as PH3 is not going to be very attractive to Venusian partners. Or not very fit over competitors who keep the scarce hydrogen for themselves.
So we should not be worried about a source pool of phosphorus in the atmosphere.
But let us assume that the report of Venusian PH3 is accurate. Where would we go on Venus to look for an environment where PH3 generation would contribute to Darwinian fitness, that is, be “biological”. As it turns out, this was not the first time that I worried about life on Venus. About 20 years ago, I sketched out a metabolism for how that life would look, in a long-forgotten white paper for NASA.
Again, Venusian life will not get water from 90% H2SO4. And, of course, the only place where fitness is improved by throwing hydrogen atoms away is an environment abundant in hydrogen. Thus, my metabolism began by finding a source of electrons able to reduce sulfuric acid to sulfurous acid, that is H2SO4 to H2SO3. The mineral pyrite does it; so does metallic iron. Deep down in fissures in the Venus crust.
Unlike sulfuric acid, sulfurous acid does not hold water aggressively at all. The reaction H2SO3 —> H2O + SO2 to give water is not bad. And from the water produced, one might extract hydrogen, and combine with phosphorus to exhale PH3. Reserving the oxygen, which is scarce in that environment.
All of this is good thought. Possibly wasted on bad data, of course. But it makes a point.
If you want to put “alien life in the gaps”, if you want to invoke life to explain a natural phenomenon, you start by asking about Darwinian fitness.

